About
Our laboratory has developed novel in-situ/operando surface analysis techniques based on scanning tunneling microscopy (STM), atomic force microscopy (AFM), X-ray absorption fine structure (XAFS), and infrared spectroscopy (IR) to visualize catalytic/electrochemical reaction processes at the atomic level (Figure 1). Such “visualization” allows us to understand what is happening on the catalyst/electrode surfaces and what factors control their performances, giving a clear guideline to improve the perfomances. We are also exploring unconventional catalytic processes using plasma and electric fields to overcome the limitations of conventional thermal catalysis.
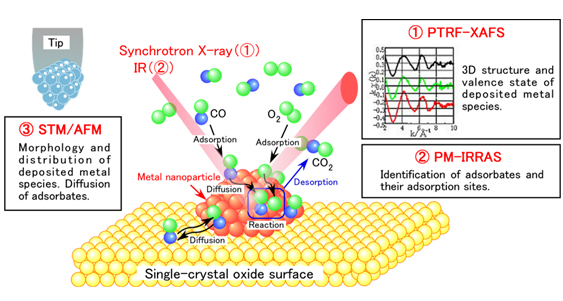
Figure 1 Atomic-level visualization of catalytic reaction processes using the “operando surface science measurement system” (①~③). The CO oxidation reaction on a model catalyst surface is shown here as an example. The reaction progress is monitored by a quadrupole mass spectrometer (QMS) to observe the catalyst surface under working conditions.
Atomic-level visualization of catalytic reaction processes
To elucidate the mechanism of surface catalytic reactions and understand the origins of catalytic activity and selectivity, it is essential to clarify the elementary steps such as
(1) where on the catalyst surface the reactant gases adsorb,
(2) how the adsorbed species (reaction intermediates) diffuse on the catalyst surface and find the active sites,
(3) what products are formed at the corresponding active sites (origin of selectivity).
Figure 2 shows the real-time STM observation of the methanol adsorption process on the Pt/TiO2(110) catalyst surface. We succeed in visualizing the spillover phenomenon for the first time in the world, where methoxy species (CH3O-Pt) formed on the Pt nanoparticles migrated to the TiO2 surface as CH3O-Ti and continued to diffuse on the TiO2 surface.
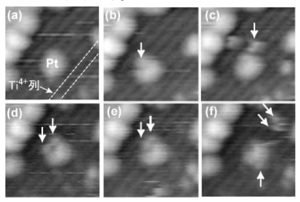
Figure 2 In situ STM observation of methanol adsorption process on Pt/TiO2(110) catalyst surface (6.4 × 6.4 nm2). The bright spots indicated by the white arrows are methoxy intermediates. 55 sec/frame.
Development of novel in situ/operando surface science techniques
Oxide-supported metal catalysts are widely used as practical catalysts. It is well known that the performance of these catalysts depends largely on the size/shape and valence state of the deposited metal species. They can change under the reaction conditions such as at high temperatures and in the presence of reactant molecules. Therefore, it is highly required to monitor them during the catalytic reactions and obtain accurate structure-activity relationship for the development of further active catalysts. Recently we have developed a novel operando surface science technique, which we call the operando polarization-dependent total reflection fluorescence (PTRF)-XAFS technique, which can provide information on the valence state (XANES) and three-dimensional (3D) structure (EXAFS) of active metal species dispersed on a well-defined oxide single-crystal surface during the catalytic reactions (Figure 3).
The developed operando PTRF-XAFS technique was applied to a Pt/α-Al2O3(0001) model catalyst during the CO oxidation reaction. Cuboctahedral Pt147 clusters were formed on the Al2O3(0001) surface under the reaction at 493 K. The turnover frequency (TOF) at 493 K was estimated to be 0.06 s–1 from simultaneous QMS and XAFS measurements. Thus, operando PTRF-XAFS has enabled the relationship between the 3D structure of a metal species on a well-defined oxide surface and its catalytic activity to be determined.
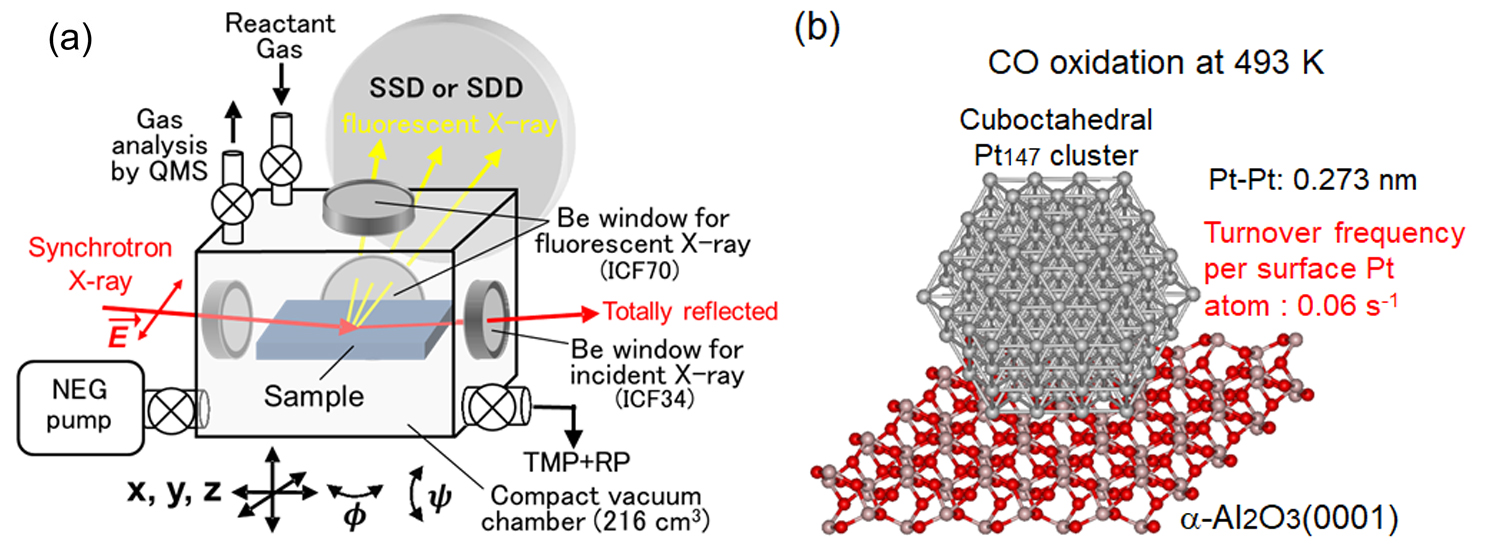
Figure 3 (a) Schematic of operando PTRF-XAFS technique. (b) 3D structure-activity relationship in CO oxidation on the Pt/α-Al2O3(0001) catalyst surface.
Plasma catalysis
Carbon neutrality is the concept of reducing overall emissions of greenhouse gases such as CO2 to zero, and various attempts are currently being made around the world to achieve this concept. Chemists are attempting to convert CO2 into useful molecules like CO and CH3OH using catalysts. However, CO2 is chemically stable, and its activation generally requires high-temperature and high-pressure conditions. On the other hand, it has recently been found that the CO2 conversion proceeds smoothly under mild conditions when combined with plasma technology. It has been suggested that the origins of the enhanced reactivity may be due to the interaction of active gas species such as vibrationally excited speies and radicals generated in the plasma with the catalyst surface, changes in the electronic state and structure of the catalyst surface induced by adsorption of active gas species, and the creation of new reaction pathways. However, the details are currently unknown. In situ/operando measurements of the catalyst surfaces in the presence of plasma are indispensable to elucidate the mechanism of the plasma-assisted catalytic reactions. We are now developing novel techniques for such measurements (Figure 4) in collaboration with researchers specializing in plasma science.
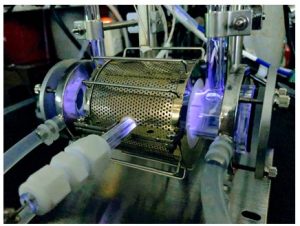
Figure 4 In situ/operando XAFS measurements during plasm-assisted catalytic reactions (BL-9A, KEK IMSS PF).
