
Research
1. Design of solid acid-base catalysts for selective conversion of biomass-derived sugars
Valorization of non-edible biomass to produce valuable chemicals represents one of the most important technologies to enable a transition to a sustainable future. Lignocellulose biomass is the most accessible “non-edible biomass resources”. We have focused on its two components (cellulose and hemicellulose) and have studied catalytic valorization of their constituents (glucose and xylose) to produce of essential chemicals (Figure 1). For example, acid-base catalysis plays a pivotal role in dehydration of glucose produces 5-hydroxymethylfurfural (HMF), while selective cleavage of carbon-carbon framework of glucose and its isomers by retro-aldol condensation leads to the formation of hydrocarbon fragments such as tetrose sugars, triose sugars, and glycolaldehyde (Figure 2). HMF is identified as versatile intermediates to produce a variety of chemicals including biomass-based plastics (vide infra). Sugar fragments with two, three and four carbons in the latter reaction can be also used as raw materials to obtain essential chemicals such as hydrocarbons (ethylene and butadiene, etc), alcohols (ethanol, ethylene glycol, propanediol, and butanediol, etc.), and carboxylic acids (lactic acid, acrylic acid, succinic acid, etc.). We have investigated Lewis acid and base properties of metal oxides in order to achieve selective conversion of glucose and its isomers to these target compounds. We reported that water-tolerant Lewis acid on amorphous Nb2O5 and Lewis acid-base pair sites on anatase TiO2 facilitates the dehydration of glucose to HMF. In contrast, Lewis acid-base pair sites on amorphous YNbO4 were active for the formation of a C3 fragment (lactic acid) from glucose.
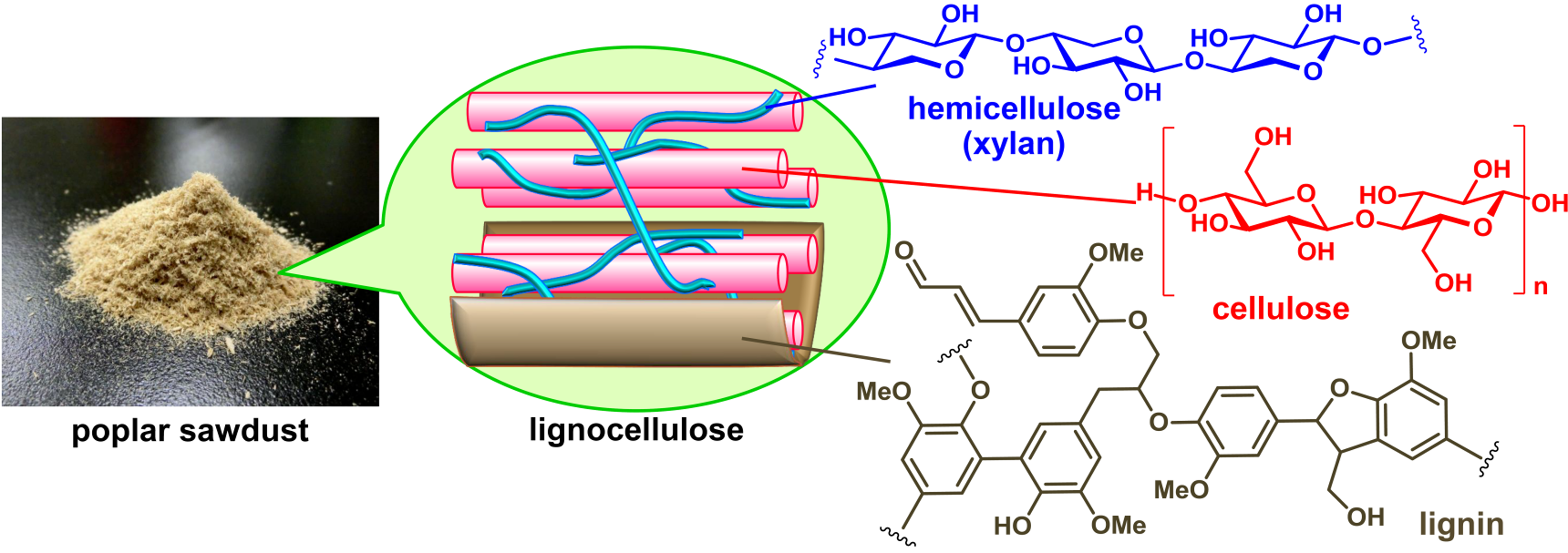
Figure 1. Structure of lignocellulose biomass
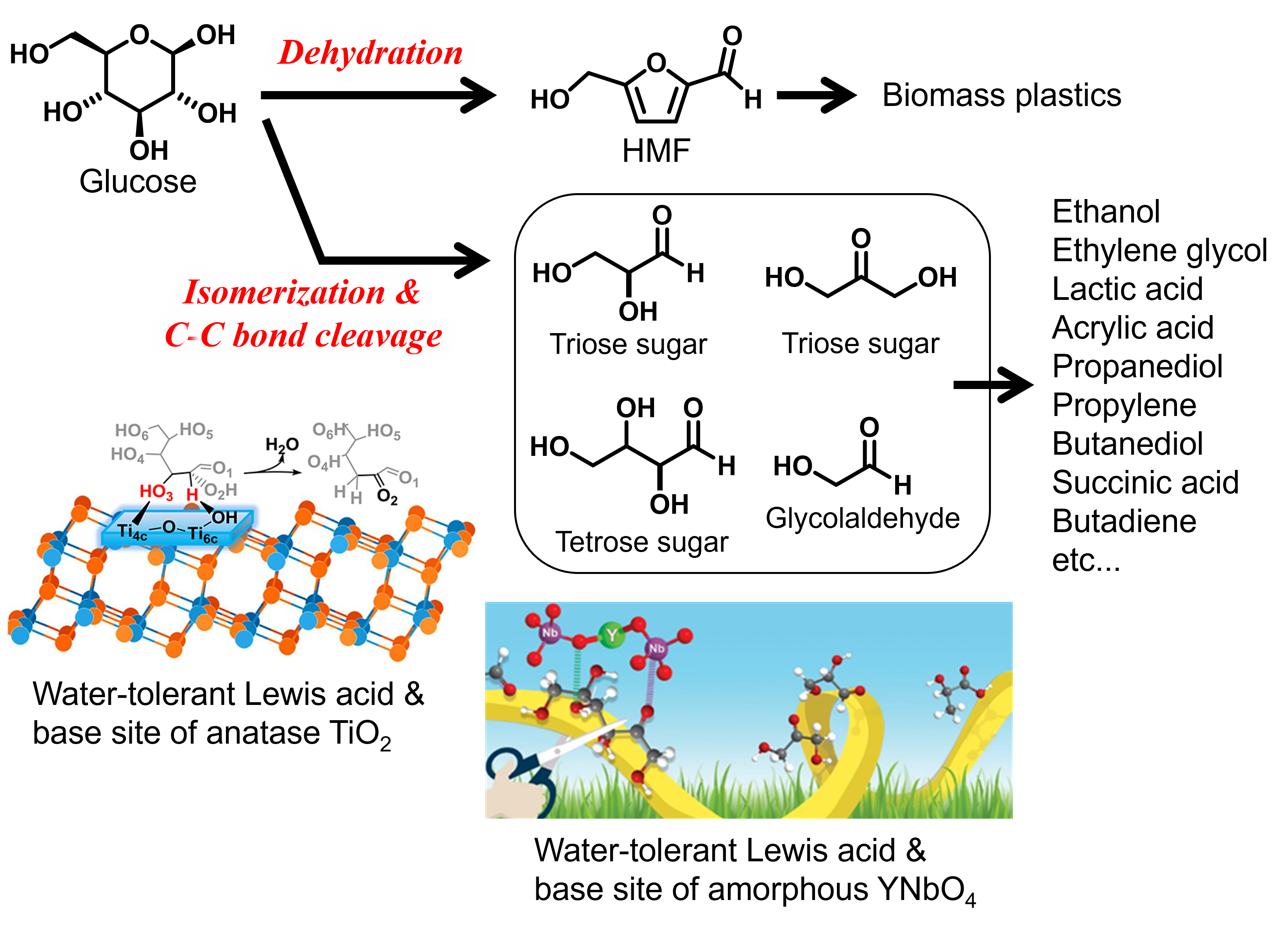
Figure 2. Conversion of glucose to platform molecules by acid-base catalysis
Related publications:
- Minjune Kim, Silvia Maria Ronchetti, Barbara Onida, Nobuyuki Ichikuni, Atsushi Fukuoka, Hideki Kato, Kiyotaka Nakajima, ChemCatChem, 2020, 12, 350-359.
- Mizuho Yabushita, Natsumi Shibayama, Kiyotaka Nakajima, Atsushi Fukuoka, ACS Catalysis, 2019, 9, 2101-2109.
- Guanna Li, Evgeny A. Pidko, Emiel J.M. Hensen, Kiyotaka Nakajima, ChemCatChem, 2018, 10, 4084-4089.
- Kiyotaka Nakajima, Jun Hirata, Minjune Kim, Navneet Kumar Gupta, Toru Murayama, Akihiro Yoshida, Norihito Hiyoshi, Atsushi Fukuoka, Wataru Ueda, ACS Catalysis, 2018, 8, 283-290.
- Toru Murayama, Kiyotaka Nakajima, Jun Hirata, Kaori Omata, Emiel J. M. Hensen and Wataru Ueda, Catalysis Science & Technology, 2017, 7, 243-250.
- Navneet Kumar Gupta, Atsushi Fukuoka, Kiyotaka Nakajima, ACS Catalysis, 2017, 7, 2430-2436.
- Ryouhei Noma, Kiyotaka Nakajima, Keigo Kamata, Masaaki Kitano, Shigenobu Hayashi, Michikazu Hara, Journal of Physical Chemistry C, 2015, 119, 17117-17125.
- Kiyotaka Nakajima, Yusuke Baba, Ryouhei Noma, Masaaki Kitano, Junko N. Kondo, Shigenobu Hayashi, Michikazu Hara, Journal of the American Chemical Society, 2011, 133, 4224-4227.
2. Catalytic conversion of biomass-derived furanics to building blocks of essential plastics
Selective conversion of the two functional groups in HMF to -OH group, -COOH group, and –NH2 group produces a variety of monomers for biomass-based plastics. However, HMF easily undergoes side reactions to form an insoluble and condensed material called humins. These side reactions are even more severe in concentrated HMF solutions (> 10 wt%). Insoluble byproduct formation in concentrated solutions hampers practical implementation of HMF utilization in chemical industry. We found that the introduction of a six-membered ring acetal with 1,3-propanediol largely suppresses humin formation during redox reactions. We have studied selective conversion of the HMF-acetal using supported metal catalysts to a variety of furanics with carboxylic acid, carboxylate, and amine group.
2,5-Furandicarboxylic acid (FDCA) synthesized by the oxidation of HMF from lignocellulose biomass is widely recognized as a suitable replacement for petroleum-derived terephthalic acid in plastics applications (Figure 4). Polymerization of FDCA with ethylene glycol forms polyethylene furanoate (PEF), which possesses physical, mechanical, and thermal properties superior to polyethylene terephthalate (PET). PEF is a promising 100% renewables-based polymer derived from plants that can replace the giant of the plastic industry, PET (global annual production >50 MT). PEF has better barrier properties and higher tensile strength than PET. Despite its great potential, efficient PEF production systems that use heterogeneous catalysts are limited to dilute solutions (≤ 5 wt%), which results in low productivity. In sharp contrast, our novel catalytic strategy involving protective chemistry enabled the selective production of FDCA from concentrated HMF-acetal solutions. We expect that our findings will become one of key technologies for the design of industrial FDCA production.
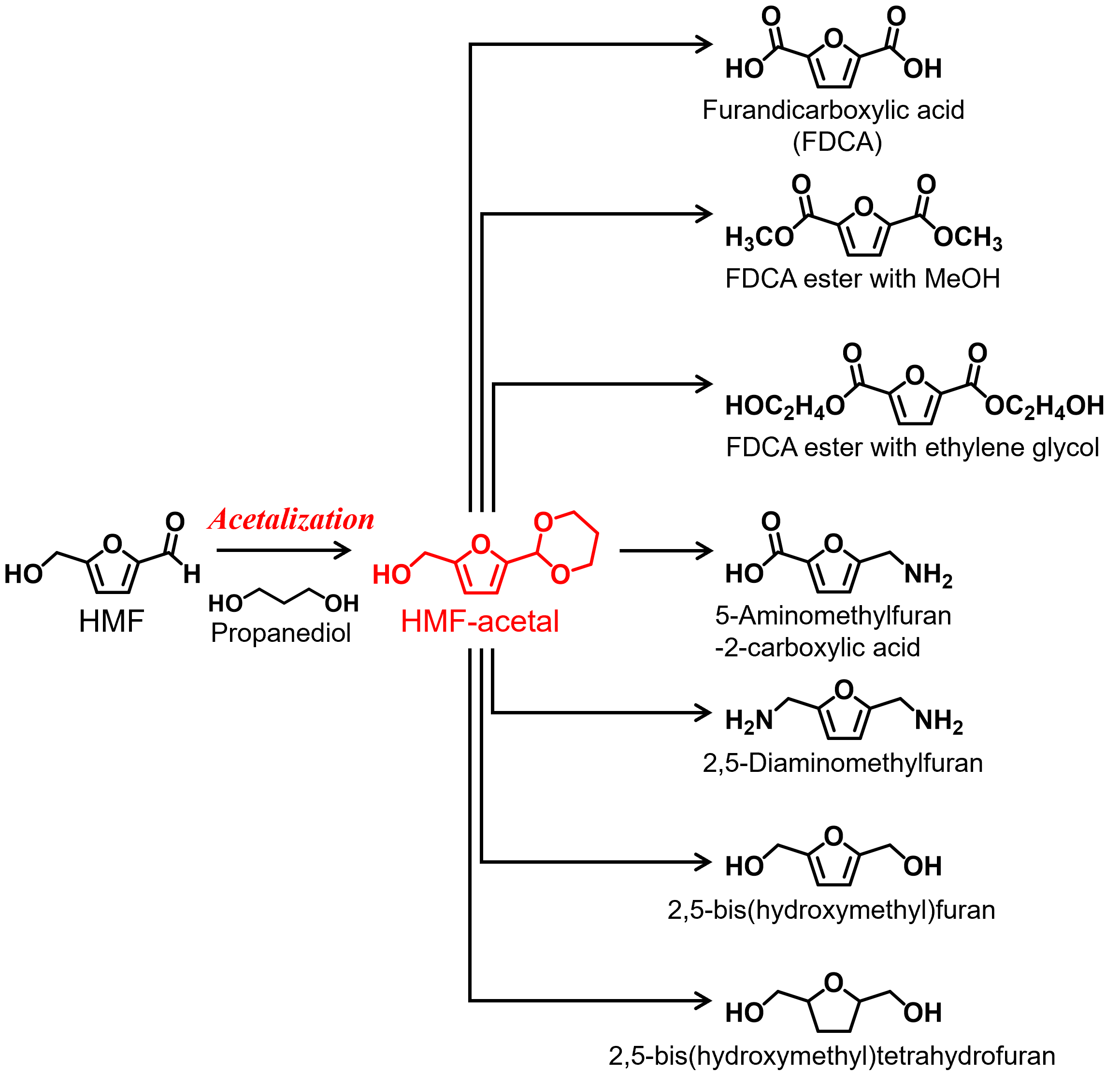
Figure 3. Conversion of glucose to platform molecules by acid-base catalysis

Figure 4. Synthetic routes for a biomass derived and highly functional polyester (PEF)
Related papers:
- Tat Boonyakarn, Jan J. Wiesfeld, Miyuki Asakawa, Lulu Chen, Atsushi Fukuoka, Emiel J. M. Hensen, Kiyotaka Nakajima, ChemSusChem, in press.
- Ferdy Coumans, Zhanna Overchenko, Jan J. Wiesfeld, Nikolay Kosinov, Kiyotaka Nakajima, Emiel J.M. Hensen, ACS Sustainable Chemistry & Engineering, in press.
- Jan J. Wiesfeld, Minjune Kim, Kiyotaka Nakajima, Emiel J.M Hensen, Green Chemistry, 2020, 22, 1229-1238.
- Minjune Kim, Yaqiong Su, Atsushi Fukuoka, Takayuki Aoshima, Emiel J. M. Hensen, Kiyotaka Nakajima, ACS Catalysis, 2019, 9, 4277−4285
- Minjune Kim, Yaqiong Su, Atsushi Fukuoka, Emiel J.M. Hensen, Kiyotaka Nakajima, Angewandte Chemie International Edition, 2018, 57, 8235-8239.
3. Studies on reaction dynamics on the surface of heterogeneous catalysts by combining advanced spectroscopies and theoretical calculations
Understanding structure of catalytically active site and its property at the molecular level is essential to sophisticate reaction systems. We are using an interdisciplinary approach that combines advanced spectroscopy, kinetic study, sometimes using isotope-labeled substrate, and theoretical calculations to understand surface reaction dynamics of catalytically active sites and reaction intermediates (Figure 5). The combination of theoretical and experimental approaches makes our proposed mechanisms more accurate. For example, we clarified that concerted catalysis of Lewis acidic TiO4 tetrahedron and Lewis basic TiOH in the second coordination shell facilitates stepwise dehydration of glucose to HMF via a unique 3-deoxyglucosone intermediate. Theoretical calculations are supported by international collaboration with TU/e (Eindhoven University of Technology, Netherlands). We encourage all members, especially PhD candidates and postdoctoral fellows, to participate in such international activities.

Figure 5. A typical research circle in Nakajima group: 1) design of heterogeneous catalysts and their catalytic reactions, 2) advanced spectroscopies, and 3) theoretical calculations
Related papers:
- Minjune Kim, Yaqiong Su, Atsushi Fukuoka, Takayuki Aoshima, Emiel J. M. Hensen, Kiyotaka Nakajima, ACS Catalysis, 2019, 9, 4277−4285
- Minjune Kim, Yaqiong Su, Atsushi Fukuoka, Emiel J.M. Hensen, Kiyotaka Nakajima, Angewandte Chemie International Edition, 2018, 57, 8235-8239.
- Guanna Li, Evgeny A. Pidko, Emiel J.M. Hensen, Kiyotaka Nakajima, ChemCatChem, 2018, 10, 4084-4089.

